Do you still register production batch records on paper or in Excel sheets as a medical device manufacturer?




.png)





Due to MDR you are obliged to have complete traceability of your operations as a medical device manufacturer.
Many medical device manufacturers still use paper or Excel to register activities during production. This is highly outdated.
Due to the manual tracking of production activities, medical device companies lose time with the administration of these activities.
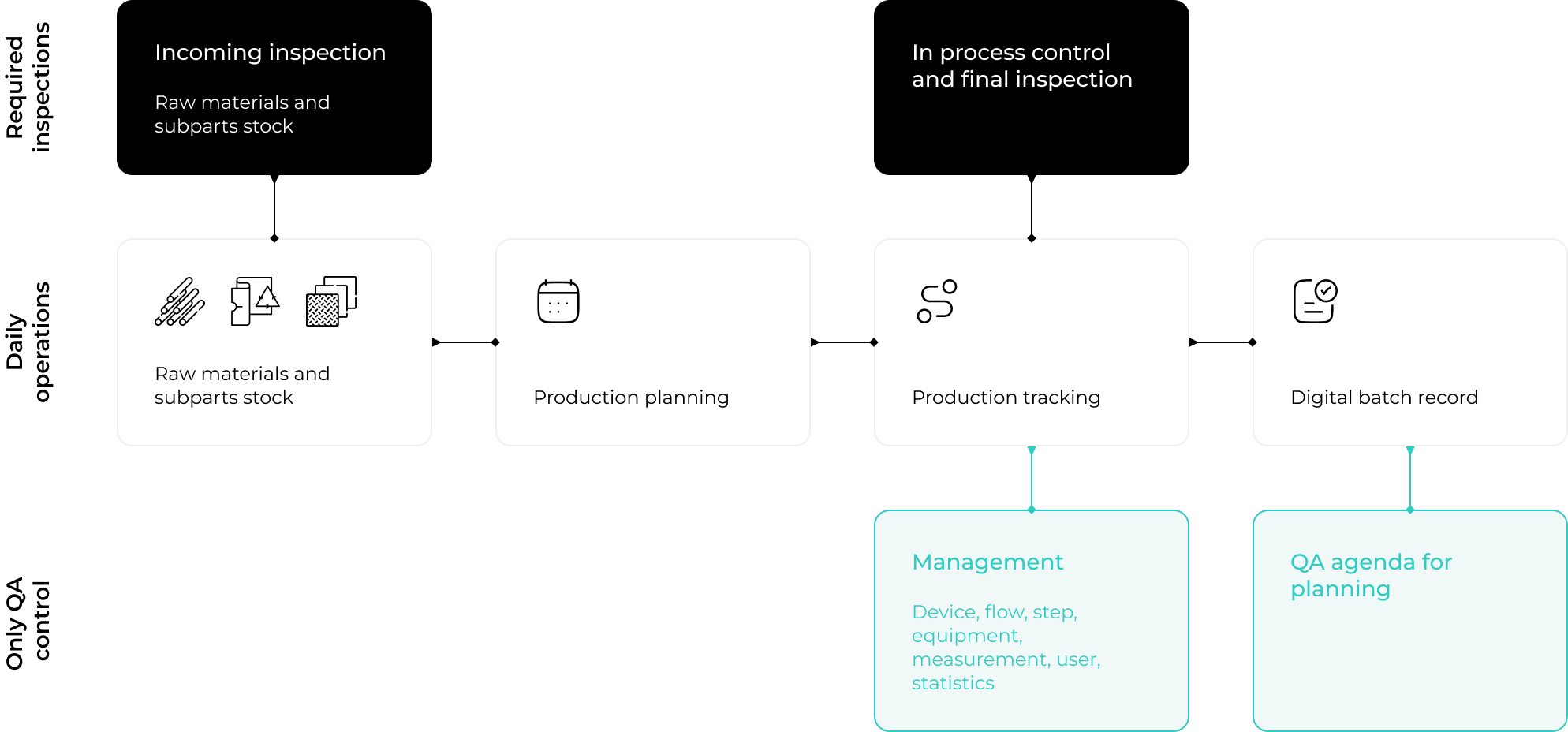
MediTrace allows you to register your entire production process as medical device manufacturer. You can manage all production related resources that eventually impact your devices. Set up your devices and production flows, and start creating digital batch records by means of QR code scanning.

With MediTrace, you can now create digital batch records by means of QR code scanning. No more running to your desk to open an Excel and register a production activity. No more printing paper batch records and manually filling it in. No, now you can simply scan your batch, scan your production step, and register every activity on the spot. This saves a lot of time, avoids mistakes, and makes your production process highly efficient.
With MediTrace, you can now manage all resources that have an impact on your medical device in one system. Manage your raw materials and processing aids with direct links to the lot numbers of your suppliers. Manage your production equipment as well as your monitoring and measuring equipment, and plan their calibration and maintenance events in a designated QA agenda.


Because now you can manage all resources in one system, MediTrace allows for multi-level traceability. What this means is that for example you could click on a certain raw material, and immediately you can see in which batch records this material has been used. The same applies for processing aids, production equipment, or even monitoring and measuring equipment. Furthermore, on the level of a subpart, you can track in which assembled batch records this subpart has been used. Recalls have never been easier.
Did you know that MediTrace was designed from the perspective of a quality engineer? It is very simple to manage your production steps, production flows and your devices in the system. Whenever you update something as a quality engineer, the system will automatically update all affected master records and keep version control. No more updating multiple master records, or losing hours and hours due to archiving. Everything updates in 1 click.














Karin Scheerlinck
CEO - Surgi-Tec
Being designed from a quality perspective, MediTrace is very easy to set up as a quality team.
We put a special focus on usability of the software because every production operator should be able to work with it.
No more hours of searching through batch records. Find anything in an instant due to our multi-level traceability.
Amaze auditors by being fully compliant on the operational level, and that while maintaining high operational efficiency.
All the data is cloud based and hosted in Europe. The solution is highly scalable and secure.
MediTrace is designed to be complementary with your current QMS system.