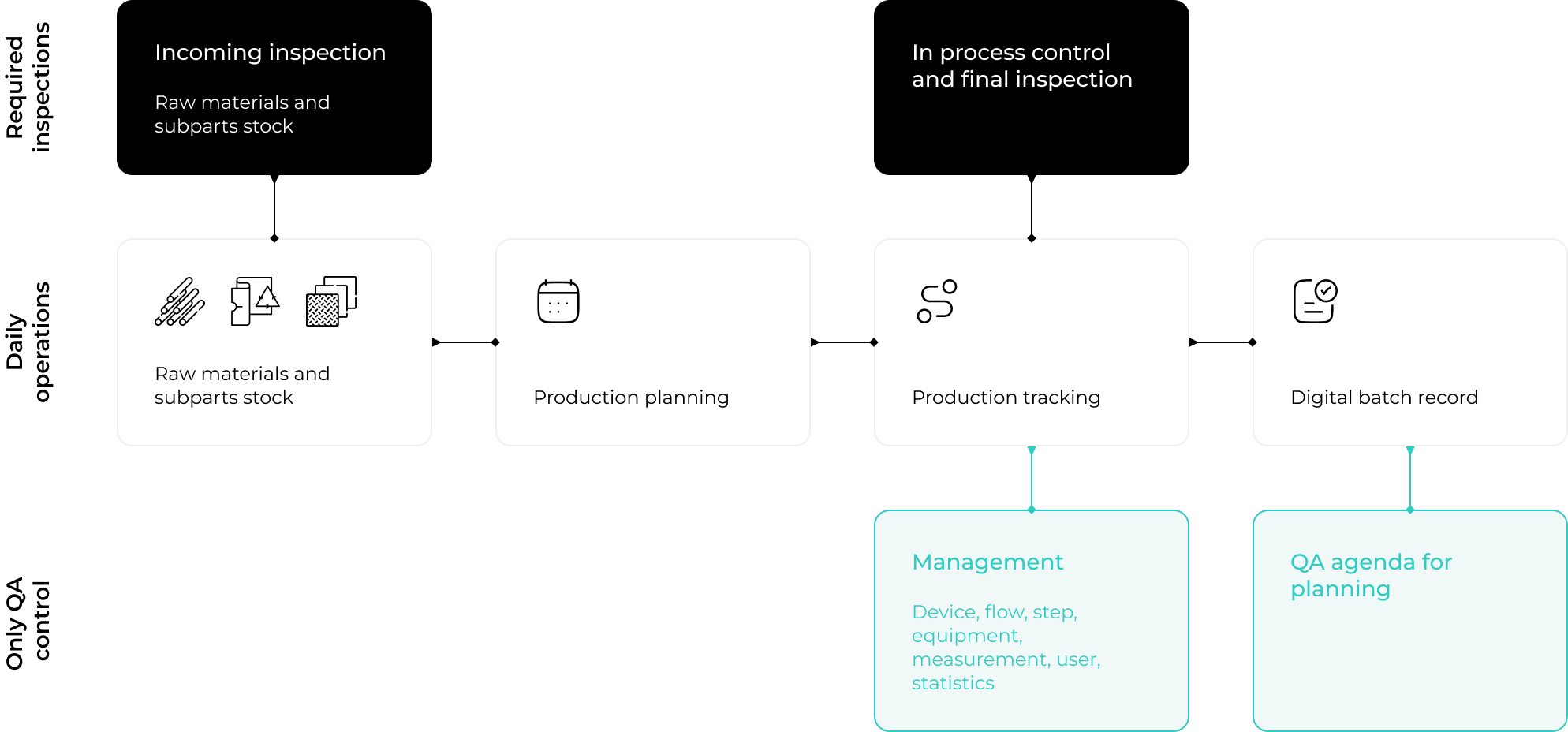
At this critical initial stage, the MediTrace software plays an essential role in ensuring the integrity of raw materials, outsourced processes, and processing aids. As these materials and services arrive, they are thoroughly inspected to confirm compliance with quality standards.
Each item is immediately logged into MediTrace, linked to its supplier’s lot number, and paired with a digitally uploaded Certificate of Conformity (CoC). This meticulous documentation process guarantees traceability and establishes a solid foundation for quality assurance right from the start.